Efficacy
Cartrophen Vet has been used in the horse for over 20 years at 2 – 2.5mg/kg (10mL vial per 400-500kg horse) and has shown to be a dependable and safe product. It is indicated as an aid in the treatment for non-infectious inflammatory joint disease including osteoarthritis.
Pentosan polysulfate is receiving recognition for its positive role in treating equine arthritis and traumatic joint disease. With many the same pathways as in dogs, Cartrophen Vet has a positive role to play in effective treatment of OA in horses. At the 2008 International Congress of World Equine Veterinary Association, Dr C McIlwraith of the University of Colorado State said the following about pentosan polysulfate.
« Recent work from our laboratory has demonstrated favourable results. Using the osteochondral fragment-treadmill model of equine OA in the carpus, there was significant decrease in articular cartilage fibrillation and a strong trend for overall cartilage histologic appearance (modified Mankin score). Furthermore, most other parameters showed numerical improvements (including lameness, joint flexion, synovial fluid, TP, synovial fluid collagen degradation products and aggrecan synthesis) although statistical significance less than 0.05 were not obtained » (McIlwraith, 2008).
Clinical Trial
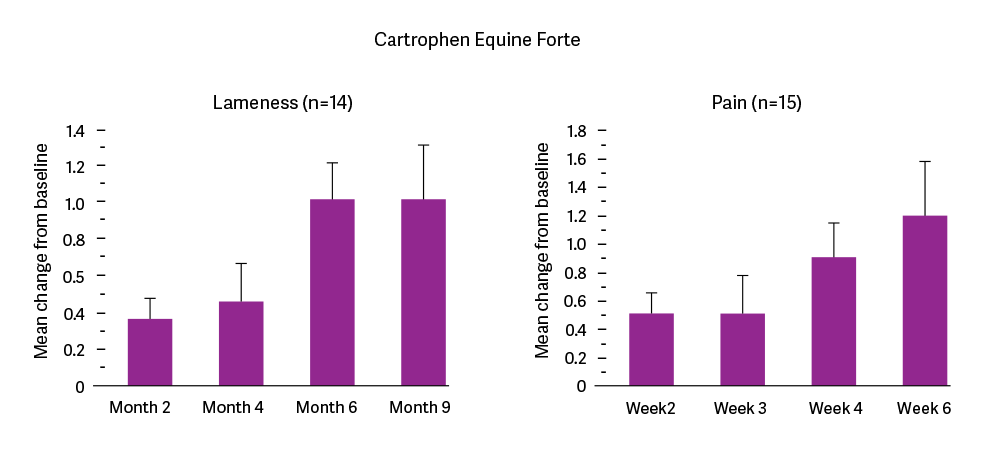
Clinical trial data shows that horses administered Cartrophen once a week for 4 weeks at an average dose of 2.2mg/kg had improved lameness and pain scores during treatment (Weeks 2, 3 and 4) and two weeks after treatment (Week 6). According to the veterinarian’s and owner’s impression of treatment, at least 60% of cases responded positively to the treatment (Data on file at Biopharm Australia Pty Ltd).
Safety
Cartrophen Vet has a history of 20 years of safe use in the horse. Based on commercial history, the number of adverse events reported in the horse on a per course basis (per 4 weekly injections) is 0.033% or one adverse event reported per 3,000 courses administered (data on file at Biopharm Australia Pty Ltd). The majority of adverse events related to poor administration technique.

